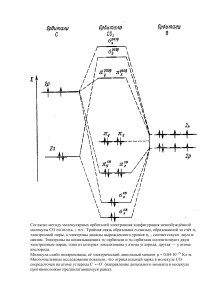
Chapter 5.3.4 Application of Hydrogen Storage Alloys Hydrogen storage alloys have unique and interesting characteristics such as a higher hydrogen storage density than that of the liquid hydrogen state, and reversible exothermic hydriding and endothermic dehydriding with fast reaction rates. Cyclic hydriding-dehydriding treatment induces pulverization of the alloy because of relatively high volume changes of the alloy by the treatment [106]. The pulverization means the reduction of the alloy particle, resulting in increasing surface area and reaction rate while decreasing heat conductivity among the particles. These phenomena should be taken into account in application [107]. Many interesting phenomena can be observed as surface and volume effects of the alloy reacting with hydrogen [107e112]. The high hydrogen storage capacity of the alloy is applied to the nickelmetal hydride (Ni-MH) rechargeable battery as commercial products. The Ni-MH battery is widely used in various scales. A small size of the Ni-MH battery is used for daily electric commodities, and the larger Ni-MH batteries are used for hybrid vehicles such as the Toyota Prius and Honda Insight. Much larger sizes of the Ni-MH batteries with megawatt (MW) capacity are used for power peak-cut and/or peak-shift of power grid, and indispensable for the efficient use of unstable renewable energy [113]. As is well known, the density of electric capacity of the Ni-MH battery is lower than the lithium (Li) ion battery. However, the Ni-MH battery is not flammable and has a high chemical stability compared with the Li ion battery. This is the main reason that many hybrid vehicles are adopting the Ni-MH battery in order to avoid firing of vehicles in accidents. Use of the Li ion battery is strictly controlled or even inhibited by mailing or air transport, as is well known. The reversible heat reactions of the alloy can be applied as a metal hydride (MH) heat pump. Unique MH freezer/water cooling systems are applied to the cultivation of strawberry in agriculture and water temperature control in fish breeding. The MH freezer technology is found markedly effective in cutting carbon emission and in saving energy compared with conventional freezing technologies using Freon gas [114]. Science and Engineering of Hydrogen-Based Energy Technologies. https://doi.org/10.1016/B978-0-12-814251-6.00014-9 290 Copyright © 2019 Elsevier Inc. All rights reserved. Application of Hydrogen Storage Alloys Chapter j 5.3.4 291 In this section, typical examples of application of hydrogen storage alloys are demonstrated in terms of the Ni-MH battery and an MH freezer system combined with waste heat. NICKEL-METAL HYDRIDE RECHARGEABLE BATTERY Typical rechargeable batteries for power storage are sodium-sulfur (NaS), redox flow (RF), Li ion and Ni-MH, and lead (Pb) cells. Typical costs per kWh of each battery are about US$400/kWh for NaS, US$ 2000/kWh for Li ion, US$ 1000/kWh for Ni-MH, and US$ 500/kWh for Pb batteries, respectively. No cost for the RF battery is given because large-scale RF batteries are still at the test stage. The RF battery, using vanadium ions for positive and negative electrodes, is attracting a strong battery at US$ 600e700/kWh. NGK Insulators, Ltd. has applied the NaS battery to MW class power storage. The NaS battery is used rather for industrial use because the battery is operated at 573K. For residential use, the Li ion battery with 7 kWh is commercialized. Typical features of the Ni-MH battery, such as high chemical stability and high cyclic charge-discharge durability under high current and load use, are confirmed by many commercialized hybrid vehicles in and outside Japan. The high diffusivity of H atoms inside the negative electrode of hydrogen storage alloys enables the high current density, and the rare earthebased hydrogen storage alloys used for the negative electrode are responsible for high chemical and cyclic stabilities. Large-scale Ni-MH batteries are used for power storage and control in transportation and residential use because of their high safety and reliability under high load. Kawasaki Heavy Industries, Ltd. has developed Ni-MH rechargeable batteries with high capacities for power storage and control (Fig. 5.47). FIGURE 5.47 Applications of the nickel-metal hydride (Ni-MH) rechargeable batteries GIGACELL (Kawasaki Heavy Industries, Ltd.) to power storage and control systems for monorail trains of Tokyo Monorail Co., Ltd. (left), and to power smoothing of a wind power system in Akita Prefecture, Japan (right) [115]. 292 Science and Engineering of Hydrogen-Based Energy Technologies Many applications using the Ni-MH rechargeable battery are demonstrating prominent results in transportation use such as light rail vehicles, trains, and hybrid vehicles; and in residential use, such as smoothing of fluctuating Renewable Energy (RE) supply. Many companies are constructing and demonstrating smart communities or towns where the Li ion and the NiMH battery are widely used for power storage and grid control. APPLICATIONS OF METAL HYDRIDE AS A FREEZER SYSTEM [114,116] Operating Principle of a Metal Hydride Freezer [116] In an MH freezer system, a pair of hydrogen storage alloys for high and low temperatures are used as shown in Fig. 5.48. In this system, waste heat from outside is needed to drive hydrogen in hydrides. A hydride of a high temperature alloy, Ma, desorbs H2 gas by absorbing waste heat Qa at TH. The desorbed H2 gas is transported to another low temperature alloy, Mb, to form a hydride MHb at room temperature TM. This exothermic hydriding reaction releases heat Qb, which is not used in this case. Then MHb desorbs H2 gas by absorbing heat Qb from the surroundings, resulting in reducing temperature of air or water from TM to a lower temperature. The H2 gas desorbed from MHb is transported back to Ma to form a hydride. By implementing this cyclic H2 gas reaction between the alloys Ma and Mb, the temperature of the surroundings of Mb cools. Using this system, different types of the MH freezer can be operated in temperature ranges below 243 K, or at 273e278 K, respectively. The temperatures can be selected and adjusted for freezing foods (meats/fishes) or for FIGURE 5.48 Principle of a metal hydride (MH) freezer system with high and low temperature hydrogen storage alloys, Ma and Mb, respectively. Application of Hydrogen Storage Alloys Chapter j 5.3.4 293 cooling agricultural products such as vegetables and fruits above freezing temperatures. An MH freezer system can be used not only for freezing, but for the production of cold water at temperatures above freezing. The latter temperatures are important to control water temperature for agriculture and fish cultivation. These technologies have been examined in various ways over 10 years at the city of Saijo, Ehime Prefecture, Japan, by the cooperation among Tokai University, Japan Steel Works (JSW), Ltd., the Ministry of Economy, Trade and Industry (METI), and Saijo City, Ehime Prefecture. Fig. 5.49 shows a plan of an MH freezer system using a high-temperature heat source of waste heat from an industry or an incinerator, and a lowtemperature heat source of ground water. Hydrogen Storage Alloys for a Metal Hydride Freezer Fig. 5.50(A) and (B) show the pressure isotherms of the developed hydrogen storage alloys, TieZreMneVeFe and TieZreCreFeeNieMneCu for highand low-temperature alloys, respectively. The amount of hydrogen transferred to drive heat reactions was over 100 cc/g-alloy at these conditions: dehydriding at 433 K and 1.0 MPa, and hydriding at 313 K and 0.075 MPa, respectively. The reduction of the maximum hydrogen storage capacity was found to be less than 2% after 10,000 hydridingedehydriding cycles. Conventional Output into Environment Hydrogen Storage Alloy Hydriding (exothermic reaction) Dehydriding (endothermic reaction) 253 k – 278 k Refrigerator Freezer and Air Conditioner etc Waste Heat utility 50 % at 276K 45% at 253K (25% at 253K by a Long Heat transport) FIGURE 5.49 Utilization of high-temperature waste heat sources of industry and ground water, respectively, for the operation of a metal hydride (MH) freezer. 294 Science and Engineering of Hydrogen-Based Energy Technologies FIGURE 5.50 Pressure isotherms of the TieZreMneVeFe (A) and the TieZreCreFeeNie MneCu and (B) for high- and low-temperature hydrogen storage alloys, respectively (JSW, Ltd.). Application of Hydrogen Storage Alloys Chapter j 5.3.4 295 FIGURE 5.51 A metal hydride (MH) freezer system (left) with control unit and two freezer rooms. Two freezer rooms (right) for 243 K and for 273e278 K with a volume of 67 m3, respectively. Fig. 5.51 show a whole MH freezer system and two freezer rooms, respectively. Each room has a volume inside 67 m3. The temperature of each room was designed for food preservation. The amounts of hydrogen storage alloys in MH tanks were 137 kg 2 and 120 kg 2 for high- and low-temperature heat reactions, respectively. The time for a cyclic operation of the alloys was around 2000 s. At temperature 283 K outside, the temperature inside a room fell to below 243K within 4 h. Realizing a temperature as low as 243 K, the low temperature alloy is cooled until 290 K using ground water. And then the alloy is cooled further using a hydrocarbon cooler (5.5 kW) until 283 K. Endothermic reactions by dehydriding of the low temperature alloy realizes a freezer temperature of 243 K after these two steps. If a groundwater or cold heat source has a temperature less than 290 K, such an additional cooler is not needed to realize 243 K. The durability of the alloys is very stable at cycles over 30,000e50,000 over 3 years’ use. Energy Consumption and CO2 Reduction The energy consumption is less than 30% that of a conventional chlorofluorocarbon-type freezer, because the MH system utilizes hightemperature waste heat from industrial facilities and low temperature of groundwater. The extra energy is required for an additional hydrocarbon cooler and for electric fans to circulate air inside the rooms. This system reduces the CO2 emission over 70% compared with a conventional CFC system. The MH freezer system is a prominent result of eco-technology, or environment-friendly green technology. This system was also examined to produce cold water for strawberry cultivation and fish breeding. In that system, the effects of CO2 reduction and energy saving were over 80%. Thus the combination of an MH freezer and high temperature waste heat is a prominent way for energy saving and CO2 reduction for freezing/cold food preservation, and environmentally friendly agriculture and fish breeding. 296 Science and Engineering of Hydrogen-Based Energy Technologies CONCLUSION Hydrogen storage alloy has highly interesting features as shown here. Many types of the alloy have been developed so far, however only limited numbers of the alloy are practically used such as rare earth base type MmNi5, magnesium-base type Mg2Ni, and titanium base type FeTi. Some alloys with specific compositions have been developed and used for a certain purpose as shown for the MH freezer system. The cost of the alloys was relatively high, however mass production of the nanostructured FeTi alloy was accomplished, and this reduced the cost by a third compared with those of conventional alloys [117]. This may accelerate use of the alloy for safe hydrogen storage in a large scale; for example, hydrogen fuel stations for fuel cell vehicles and effective control of fluctuating electric generation by renewable energy. Many more extended applications of the alloy are expected. CONCLUDING REMARKS Traditionally, hydrogen production has been using fossil fuels, but in recent years technologies that can be manufactured with renewable energy have been developed and expected to be high. Fossil fuels are used for most of the hydrogen produced, but hydrogen production by renewable energy is expected to develop in the future. Hydrogen production that does not emit carbon dioxide is an important development item because it can reduce the impact on global warming. Hydrogen production including electrolysis will become mainstream in the future. In addition, demonstration projects of fuel cell vehicles have been carried out many times in Europe, America, and Asia in the 21st century. As a result, fuel cell vehicles were sold and fuel cell buses also began to run on ordinary roads. Production volume of these fuel cell vehicles surely grows, and it seems that it will occupy a part of mainstream automobiles in the future. In addition, hydrogen refueling stations have been under construction since 2015 in Japan, and now more than 100 hydrogen stations have been built. It is planned that 160 places will be built in 2020 and about 900 stations will be constructed by 2030. Hydrogen energy technology is definitely beginning to penetrate society. Because concern about global warming due to carbon dioxide has increased, a technique of reacting hydrogen with carbon dioxide is attracting attention as hydrogen utilization technology. Although this is a challenging study, it will be developed as a technology to be incorporated into the chemical process. Furthermore, the use of hydrogen storage alloy is promoted as an energy stockpile. Hydrogen energy plants using storage alloys have been commercialized in recent years. Hydrogen storage is a technology that enables clean energy on a yearly basis, and it can be regarded as a distinguishing technology. Application of Hydrogen Storage Alloys Chapter j 5.3.4 297 In this way, hydrogen energy technology is beginning to penetrate into society and has made remarkable progress. In the future, further growth in this field will continue. REFERENCES [1] [2] [3] [4] [5] [6] [7] [8] [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] [20] M. Asif, T. Muneer, Energy supply, its demand and security issues for developed and emerging economies, Renew. Sustain. Energy Rev. 11 (2007) 1388e1413. T. Muneer, M. Asif, J. Kubie, Generation and transmission prospects for solar electricity: UK and global markets, Energy Convers. Manag. 44 (2003) 35e52. T.N. Veziroglu, Hydrogen movement and the next action: fossil fuels industry and sustainability economics, Int. J. Hydrogen 22 (1997) 551e556. Hydrogen Scaling Up - A sustainable pathway for the global energy transition, Hydrogen Council, November 2017. http://hydrogencouncil.com/wp-content/uploads/2017/11/ Hydrogen-scaling-up-Hydrogen-Council.pdf. M.A. Pena, J.P. Gómez, J.L.G. Fierro, New catalytic routes for syngas and hydrogen production, Appl. Catal. Gen. 144 (1996) 7e57. I. Dincer, C. Zamfirescu, Sustainable hydrogen production, Elsevier (2016). J.W. Chun, R.G. Anthony, Partial oxidation of methane, methanol, and mixtures of methane and methanol, methane and ethane, and methane, carbon dioxide, and carbon monoxide, Ind. Eng. Chem. Res. 32 (5) (1993) 788e795. S.H.D. Lee, D.V. Applegate, S. Ahmed, S.G. Calderone, T.L. Harvey, Hydrogen from natural gas: part Idautothermal reforming in an integrated fuel processor, Int. J. Hydrogen 30 (2005) 829e842. S. Ahmed, D.D. Papadias, S.H.D. Lee, R.K. Ahluwalia, Autothermal and Partial Oxidation Reformer-Based Fuel Processor, Method for Improving Catalyst Function in Autothermal and Partial Oxidation Reformer-Based Processors, United States Patent 20100095590, 2010, p. 487. C.H. Bartholomew, Mechanisms of catalyst deactivation, Appl. Catal. 212 (2001) 17e60. R.T.K. Baker, Catalytic growth of carbon filaments, Carbon 27 (3) (1989) 315e323. M.S. Kim, N.M. Rodriguez, R.T.K. Baker, The interaction of hydrocarbons with copper nickel and nickel in the formation of carbon filaments, J. Catal. 131 (1) (September 1991) 60e73. F. Abild-Pedersen, J.K. Nørskov, J.R. Rostrup-Nielsen, J. Sehested, S. Helveg, Mechanisms for catalytic carbon nanofiber growth studied by ab initio density functional theory calculations, Phys. Rev. B 73 (20 March 2006) 115419. C.P. Forzatti, L. Lietti, Catalyst deactivation, Catal. Today 52 (1999) 165e181. J.R. Rostrup-Nielsen, New aspects of syngas production and use, Catal. Today Vol. 63 (2e4) (2000) 159. J.R. Rostrup-Nielsen, T. Rostrup-Nielsen, Large-scale hydrogen production, CATTECH 6 (4) (August 2002) 150e159. (a) http://www.jfecon.jp/product/hpc/fcv.html. (b) http://www.hydrogencarsnow.com/index.php/hydrogen-fuel-tanks/. T. Nejat, Transportation fuel hydrogen, Energy Technol. Environ. 4 (1995) P2712eP2730. Grace Odaz et al., Hydrogen Storage: 2005 Annual DOE Hydrogen. K.T. Møllera, T.R. Jensen, E. Akiba, H.-W. Li, Prog. Nat. Sci. Mater. Int. 27 (2017) 34e40. 298 Science and Engineering of Hydrogen-Based Energy Technologies [21] [22] [23] [24] [25] [26] [27] [28] [29] [30] [31] [32] [33] [34] [35] [36] [37] [38] [39] (a) http://www.jari.or.jp/portals/0/jhfc/station/index.html. (b) www.airliquide.com/united-states-america/air-liquide-announces-locations-severalhydrogen-fueling-stations-northeast. (c) California Fuel Cell Partnership OEM Advisory Group. Letter to Hydrogen Infrastructure Stakeholders. June 11, 2014. TS. http://cafcp.org/getinvolved/stayconnected/ blog/automakers_release_list_station_priority_locations. (d) http://media.daimler.com/marsMediaSite/en/instance/ko/First-hydrogen-filling-stationopens-in-Bremen.xhtml?oid¼29897600. H2-Mobility Hydrogen Station. http://h2-mobility.de/en/h2-stations/. https://www.iea.org/publications/freepublications/publication/TechnologyRoadmapHydrogen andFuelCells.pdf. K. Mazloomi, C. Gomes, Hydrogen as an energy Carrier: prospects and challenges, Renew. Sustain. Energy Rev. 16 (5) (June 2012) 3024e3033. J. Gretz, J.P. Baselt, O. Ullmann, H. Wendt, The 100 MW euro-Quebec hydro-hydrogen pilot project, Int. J. Hydrogen Energy 15 (6) (1990) 419. J. Gretz, J.P. Baselt, D. Kluyskens, F. Sandmann, O. Ullmann, Status of the hydrohydrogen pilot project (EQHHPP), Int. J. Hydrogen Energy 19 (2) (1994) 169. K. Jouthimurugesan, A.K. Nayak, G.K. Metha, K.N. Rai, S. Bhatia, R.D. Srivastava, Role of rhenium in Pt-Re-Al2O3 reforming catalysisdan integrated study, AIChE J. 31 (12) (1985) 1997e2007. K. Jouthimurugesan, S. Bhatla, R.D. Srivastava, Kinetics of dehydrogenation of methylcyclohexane over a platinum-rhenium-alumina catalyst in the presence of added hydrogen, Ind.. Eng. Chem. Fundam. 24 (4) (1985) 433. R.W. Coughlin, K. Kawakami, A. Hasan, Activity, yield patterns, and coking behavior of Pt and PtRe catalysts during dehydrogenation of methylcyclohexane: I. In the absence of sulfur, J. Catal. 88 (1984) 150. W.J. Doolittle, N.D. Skoularikis, R.W. Coughlin, Reactions of methylcyclohexane and n-heptane over supported Pt and PtRe catalysts, J. Catal. 107 (2) (1987) 490. M. Markiewicz, Y.Q. Zhang, A. Bösmann, N. Brückner, J. Thöming, P. Wasserscheid, S. Stolte, Environmental and health impact assessment of Liquid Organic Hydrogen Carrier (LOHC) systems e challenges and preliminary results, Energy Environ. Sci. 8 (2015) p1035e1045. A. Ozaki, K. Aika, Catalysis, science and technology, in: J.R. Anderson, M. Boudart (Eds.) Vol. 1, Springer Verlag, Berlin, 1981, p. 88 (Chapter 3). https://minerals.usgs.gov/minerals/pubs/mcs/2018/mcs2018.pdf. S.R. Tennison, Catalytic ammonia synthesis: fundamentals and practice, in: J.R. Jennings (Ed.), Fundamental and Applied Catalysis, Plenum Press, New York, 1991. M. Boudart, G. Djéga-Mariadassou, Kinetics of Heterogeneous Catalytic Reactions, Princeton University Press, Princeton, 1984. S.R. Tennison, Catalytic ammonia synthesis: fundamentals and practice, in: J.R. Jennings (Ed.), Fundamental and Applied Catalysis, Plenum Press, New York, 1991. F.M. Hoffmann, D.J. Dwyer, Surface science of catalysis: in situ probes and reaction kinetics, in: D.J. Dwyer, F.M. Hoffmann (Eds.) Vol. 482, American Chemical Society, Washington, DC, 1991, p. 1 (Chapter 1). https://ammoniaindustry.com/download-salient-ammonia-statistics-usgs/. H. Yan, Y.-J. Xu, Y.-Q. Gu, H. Li, X. Wang, Z. Jin, S. Shi, R. Si, C.-J. Jia, C.-H. Yan, Promoted multimetal oxide catalysts for the generation of hydrogen via ammonia decomposition, J. Phys. Chem. C 120 (2016) 7685e7696. Application of Hydrogen Storage Alloys Chapter j 5.3.4 [40] [41] [42] [43] [44] [45] [46] [47] [48] [49] [50] [51] [52] [53] [54] [55] [56] [57] [58] [59] [60] [61] [62] [63] 299 A. Boisen, S. Dahl, J.K. Nørskov, C.H. Christensen, Why the optimal ammonia synthesis catalyst is not the optimal ammonia decomposition catalyst, J. Catal. 230 (2) (March 10, 2005) 309e312. C. Jacobsen, S. Dahl, B.S. Clausen, S. Bahn, A. Logadottir, J.K. Nørskov, Catalyst design by interpolation in the periodic Table: bimetallic ammonia synthesis catalysts, J. Am. Chem. Soc. 123 (34) (2001) 8404e8405. JHFC was a Demonstration Project Regarding FCVs Implemented From FY2002 to FY2010. http://www.jari.or.jp/Portals/0/jhfc/e/jhfc/index.html. Toyota Envisions the Fuel Cell Bus of The Future. https://newatlas.com/toyota-sora-fuelcell-bus-tokyo/51825/. Toyota Mirai Fuel Cell Vehicle, The Future, 2017. Available from: https://ssl.toyota.com/ mirai/fcv.html. Policy Research Institute for Land, Infrastructure, Transport and Tourism. Ministry of Land, Infrastructure, Transport and Tourism Search Japanese. http://www.mlit.go.jp/pri/ english/houkoku/gaiyou/english_kkk59.html. L. Eudy, M. Post, M. Jeffers, Fuel Cell Buses in U.S. Transit Fleets: Current Status 2016, National Renewable Energy Laboratory, November 2016. Prepared under Task No. HT12.8210, Technical Report, NREL/TP-5400-67097, Contract No. DE-AC36-08GO28308. The Association of Hydrogen Supply and Utilization Technology HySUT. http://hysut.or. jp/en/index.html. Hydrogen Station. http://fccj.jp/hystation/. Hydrogen Station. https://www.navitime.co.jp/category/0818/. Hydrogen Station. http://toyota.jp/mirai/station/. Y. Matsuda, et al., JARI Res. J. 32 (7) (2010) 345e348. ISO FDIS 14687-2. International Standardization for Hydrogen Fuel for Fuel Cell Vehicles. Hydrogen Fueling Infrastructure Analysis. https://www.nrel.gov/hydrogen/hydrogeninfrastructure-analysis.html. A California Road Map. http://www.fuelcellpartnership.org/carsandbuses/caroadmap/ Overview-of-FCH2-Developments-in-Germany.pdf. Towards sustainable energy systems eOverview of German FC-Developments http:// www.iphe.net/docs/Meetings/SC21/Educational%20Event%20Presentations/Overview-ofFCH2-Developments-in-Germany.pdf. Scenario for FCV and Hydrogen Station Dissemination. http://fccj.jp/pdf/22_cse.pdf. UK H2 Mobile “UK H2 Mobile Phase1 Result”. http://www.iphe.net/docs/Meetings/SC21/ Educational%20Event%20Presentations/Overview-of-FCH2-Developments-in-Germany.pdf. H2USA Home-Page. http://h2usa.org/. H2 Mobility Press Release. http://www.now-gmbh.de/en/presse-aktuelles/2013/h2-mobilityinitiative.html. Hydrogen Society in Japan Plane. http://www.meti.go.jp/english/press/2014/0624_04.html. A.H. Kakaee, A. Paykani, M. Ghajar, The influence of fuel composition on the combustion and emission characteristics of natural gas fueled engines, Renew. Sus. Energ. Rev. 38 (2014) 64e78. G.A. Richards, M.M. McMillian, R.S. Gemmen, W.A. Rogers, S.R. Cully, Issues for lowemission, fuel-flexible power systems, Prog. Energy Combust. Sci. 27 (2) (2001) 141e169. R.L. Bannister, R.A. Newby, W.C. Yang, Final report on the development of a hydrogenfuelde combustion turbine cycle for power generation, J. Eng. Gas Turbines Power 121 (1999) 38e45. 300 Science and Engineering of Hydrogen-Based Energy Technologies [64] [65] [66] [67] [68] [69] [70] [71] [72] [73] [74] [75] [76] [77] [78] [79] [80] [81] [82] [83] K.J. Bosschaart, L.P.H. de Goey, The laminar burning velocity of flames propagating in mixtures of hydrocarbons and air measured with the heat flux method, Combust. Flame 136 (3) (2004) 261e269. W. Lowry, J. de Vries, M. Krejci, E. Petersen, Z. Serinyel, W. Metcalfe, H. Curran, G. Bourque, Laminar flame speed measurements and modeling of pure alkanes and alkane blends at elevated pressures, J. Eng. Gas Turbines Power Trans. ASME 133 (9) (2011). E. Bancalari, I.S. Diakunchak, G. McQuiggan, A review of W501G engine design, development and field operating experience, in: ASME Paper GT 2003e38843, 2003. MITI, Comprehensive approach to the new sunshine program which supports the 21st century (Agency of Industrial Science and Technology in Ministry of International Trade and Industry [MITI]), Sunshine J. 4 (1993) 1e6. NEDO, International Clean Energy Network Using Hydrogen Conversion (WE-NET), 1993 Annual Summary Report on Results, New Energy and Industrial Technology Development Organization [NEDO], 1994. NEDO, Subtask 8 e development of hydrogen-combustion turbine, study for an optimum system for hydrogen combustion turbine, in: 1995 Annual Technical Results Report, New Energy and Industrial Technology Development Organization [NEDO], 1996. G.A. Geoffroy, D.J. Amos, Four years operating experience update on a coal gasification combined cycle plant with two 100 MW class gas turbines, in: Presented at Combined Heat and Power Independent Producers Conference, Birmingham, England, 1991. J. Karg, G. Haupt, B. Wiant, IGCC plants provide clean and efficient power using refinery residues and coal, in: Electric Power Conference, Cincinnati, Ohio, 2000. a N. Kizuka, et al., Conceptual design of the cooling system for 1700 C-class, hydrogenfueled combustion gas turbines, Trans. ASME 121 (1999) 108e115; b H. Jericha, O. Starzer, M. Theissing, Towards a Solar-Hydrogen System, in: ASME Cogen Turbo, vol. 6, IGTI, 1991, pp. 435e442. F. Hannemann, B. Koestlin, G. Zimmermann, H. Morehead, F.G. Peña, Pushing forward IGCC technology at siemens, in: 2003 Gasification Technologies Conference, San Francisco, California, 2003. http://www.nedo.go.jp/news/press/AA5_100596.html https://green.blogs.nytimes.com/2009/08/17/a-hydrogen-power-plant-in-italy/. GE Internal Study, 2007 and EPRI IGCC Design Considerations for CO2 Capture: Engineering and Economic Assessment of IGCC Coal Power Plants for near-term Deployment, 2008. https://ja.scribd.com/document/46732183/GE-Hydrogen-Fueled-Turbines. http://www.iea-coal.org.uk/documents/82227/7239/Gas-turbine-technology-for-syngas/ hydrogen-in-coal-based-IGCC. http://www.nedo.go.jp/english/publications_reports_index.html. http://www.mhi.co.jp/technology/review/indexj-54-3.html. J. Gao, A. Hossain, Y. Nakamura, Flame base structures of micro-jet hydrogen/methane diffusion flames, Proc. Combust. Inst. 36 (2017) 4209e4216. K. Kuwana, S. Kato, A. Kosugi, T. Hirasawa, Y. Nakamura, Experimental and theoretical study on the interaction between two identical micro-slot diffusion flames: burner pitch effects, Combust. Flame 165 (2016) 346e353. K. Zhao, D. Cui, T. Xu, Q. Zhou, S. Hui, H. Hu, Effects of hydrogen addition on methane combustion, Fuel Proces. Technol. 89 (11) (November 2008) 1142e1147. Application of Hydrogen Storage Alloys Chapter j 5.3.4 [84] [85] [86] [87] [88] [89] [90] [91] [92] [93] [94] [95] [96] [97] [98] [99] [100] [101] [102] [103] 301 Q. Ma, Q. Zhang, Jiachen Chen, Ying Huang, Yuantong Shi, Effects of hydrogen on combustion characteristics of methane in air, Int. J. Hydrogen Energy 39 (21) (July 15, 2014) 11291e11298. M. Klelle, H. Eichlseder, M. Sartory, Mixtures of hydrogen and methane in the internal combustion engine e synergies, potential and regulations, Int. J. Hydrogen Energy 37 (15) (August 2012) 11531e11540. M.C. Lee, S.B. Seo, J.H. Chung, Si M. Kim, Y.J. Joo, D.H. Ahn, Gas turbine combustion performance test of hydrogen and carbon monoxide synthetic gas, Fuel 89 (7) (July 2010) 1485e1491. O. Kurata, N. Iki, T. Matsunuma, T. Inoue, T. Tsujimura, H. Furutani, H. Kobayashi, A. Hayakawa, Performances and emission characteristics of NH3-air and NH3-CH4-air combustion gas-turbine power generations, Proc. Combust. Inst. 36 (2017) 3351e3359. Y. Arakawa, A. Hayakawa, K.D.K.A. Somarathne, T. Kudo, H. Kobayashi, Flame characteristics of ammonia and methane flames in a swirl combustor, in: Proceedings of 12th International Conference on Flow Dynamics (12th ICFD), Sendai, Japan, October 27e29, 2015. R. Murai, R. Omori, R. Kano, Y. Tada, H. Higashino, N. Nakatsuka, J. Hayashi, F. Akamatsu, K. Iino, Y. Yamamoto, The radiative characteristics of NH3/N2/O2 nonpremixed flame in 10 kW test furnace, Energy Procedia 120 (2017) 325e332. Mixed combustion of pulverized coal and ammonia, <http://www.denken.or.jp/en/index. html>. N. Nakatsuka, J. Fukui, K. Tainaka, H. Higashino, J. Hayashi, F. Akamatsu, Detailed observation of coal-ammonia Co-combustion processes, in: AIChE Annual Meeting, Accepted, 2017. J. Nakamura, Ghokubai no Jiten ISBN978-4-254-25242-2 C3558 P545 (2000). https://energy.gov/sites/prod/files/Elem_Coal_Studyguide.pdf. Canonico, S., Sellman, R., Preist, C., As published in the Proceedings of the 2009 IEEE International Symposium on Sustainable Systems and Technology (ISSST), ISBN 978-1244-3456-5, IEEE, 2009 Reducing the Greenhouse Gas Emissions of Commercial Print With Digital Technologies. J.H. Shinfelt, in: J.R. Anderson, M. Boudart (Eds.), Catalysis, Science and Technology vol. 1, Springer, Berlin, Heidelberg, New York, 1981, p. 257. G.C. Chinchen, P.J. Denny, et al., Review: synthesis of ethanol, Part 1 catalysts and kinetics, Appl. Catal. 36 (1988) 1e65. T. Chang, R.W. Rousseau, P.K. Kilpatrick, Methanol synthesis reactions: calculations of equilibrium conversions using equtions of states, Ind. Chem. Process Des. Dev. 25 (1986) 477e481. K. Klier, et al., J. Catal. 74 (1982) 343. H.F. Rase, Hand Book of Commercial Catalysts, Heterogeneous Catalysts, CRC Press, Boca Raton, FL, 2000. Patent WO2013183577 A1 PCT/JP2013/065325, Mitsui Chemical. Inc. B.B. Pearce, M.V. Twigg, C. Woodwrd, Catalyst Handbook Twigg, Wolfe, London, 1989, pp. 340e378 (Chapter 7). T. Inui, Methanation, in: I.T. Horvath (Ed.), Encyclopedia of Catalysis, vol. 5, John Wiley & Sons, New Jersey, 2003, pp. 51e61. E. Iglesia, Isotopic and kinetic assessment of the mechanism of reactions of CH4 with CO or H2O to form synthesis gas and carbon on Ni catalysis, J. Catal. 244 (2004) 370e383. 302 Science and Engineering of Hydrogen-Based Energy Technologies [104] [105] [106] [107] [108] [109] [110] [111] [112] [113] [114] [115] [116] [117] Catalyst for Production of Hydrocarbons, U.S. Patent 4,801,573. S. Rittmann, A. Seifert, C. Herwig, Essential prerequisites for successful bioprocess development of biological CH4 production from CO2 and H2, Crit. Rev. Biotechnol 35 (2) (Jun 2015) 141e151. H. Uchida, H.H. Uchida, Y.C. Huang, J. Less-Common Met. 101 (1984) 459e468. H. Uchida, K. Terao, Y.C. Huang, Z. Phys. Chem. N.F. 164 (1989) 1275e1284. H. Uchida, Y. Ohtani, M. Ozawa, T. Kawahata, T. Suzuki, J. Less-Common Met. 172e174 (1991) 983e996. Y. Ohtani, S. Hashimoto, H. Uchida, J. Less-Common Met. 172e174 (1991) 841e850. S. Seta, H. Uchida, J. Less-Common Met. 231 (1995) 448e453. H. Uchida, S. Seki, S. Seta, J. Less-Common Met. 231 (1995) 403e410. H. Uchida, Int. J. Hydrogen Energy 24 (1999) 861e869. H. Uchida, Japan’s energy policy after the 3.11 natural and nuclear disasters e from the viewpoint of the R&D of renewable energy and its current state (chapter 2), in: D. Stolten, V. Scherer (Eds.), Energy Transition to Renewable Energy, Wiley VCH, Germany, June 2013, pp. 109e125. ISBN: 978-7-527-67390-2. K. Ochi, K. Itoh, H. Uchida, Proc.. The18th WHEC 2010, Essen Germany vols. 78e4, Forschnungszentrum Juelich GmbH, Germany, 2010, pp. 377e384. ISBN: 978-3-89336654-5. Kawasaki Heavy Industry, GIGACELL, Railway Battery Power System for Tokyo Monorail, 2012. H. Uchida, An metal hydride refrigerator using hydrogen storage alloys, Refrigerator (REITOU) 79 (2004) 837e840. T. Haraki, K. Oishi, H. Uchida, Y. Miyamoto, M. Abe, T. Kokaji, S. Uchida, Int. J. Mater. Res. 99 (2008) 507e512.